Specific Heat And Calorimetry Worksheet Answers . Find the equilibrium temperature if 200.0 grams of cold water. ch 16 specific heat problems: The temperature of 335 g of water changed from 24.5oc to 26.4oc. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry and specific heat calculation worksheet. chemistry 122 specific heat and calorimetry worksheet 1. specific heat and heat capacity worksheet. A calorimeter has a heat capacity of 4.18 kj/g oc. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,.
from www.studocu.com
1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. calorimetry and specific heat calculation worksheet. chemistry 122 specific heat and calorimetry worksheet 1. Find the equilibrium temperature if 200.0 grams of cold water. A calorimeter has a heat capacity of 4.18 kj/g oc. ch 16 specific heat problems: The temperature of 335 g of water changed from 24.5oc to 26.4oc.
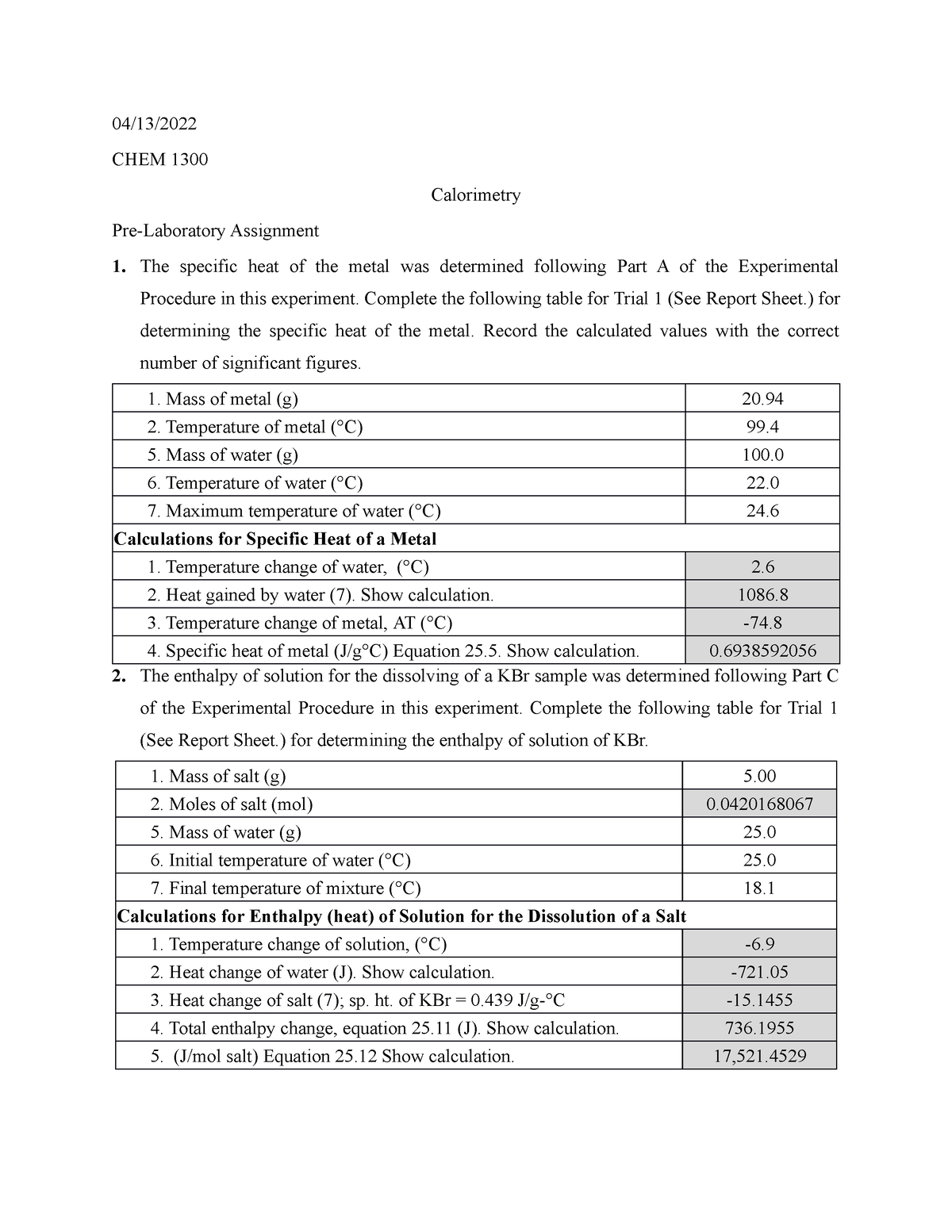
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory
Specific Heat And Calorimetry Worksheet Answers calorimetry and specific heat calculation worksheet. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of. chemistry 122 specific heat and calorimetry worksheet 1. Find the equilibrium temperature if 200.0 grams of cold water. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. specific heat and heat capacity worksheet. A calorimeter has a heat capacity of 4.18 kj/g oc. ch 16 specific heat problems: calorimetry and specific heat calculation worksheet. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. The temperature of 335 g of water changed from 24.5oc to 26.4oc.
From learningnovotyzy.z21.web.core.windows.net
Heat And Calorimetry Worksheets Specific Heat And Calorimetry Worksheet Answers Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. specific heat and heat capacity worksheet. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. ch 16 specific heat problems: A calorimeter has a heat capacity of 4.18 kj/g oc. in this set of practice questions, we will go. Specific Heat And Calorimetry Worksheet Answers.
From thekidsworksheet.com
Specific Heat Worksheet 2 Answer Key Thekidsworksheet Specific Heat And Calorimetry Worksheet Answers calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of. specific heat and heat capacity worksheet. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. The temperature of 335 g of water changed. Specific Heat And Calorimetry Worksheet Answers.
From www.worksheeto.com
15 Best Images of Specific Heat Worksheet Answer Key Specific Heat Specific Heat And Calorimetry Worksheet Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. chemistry 122 specific heat and calorimetry worksheet 1. calorimetry and specific heat calculation worksheet. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of. ch 16. Specific Heat And Calorimetry Worksheet Answers.
From lessoncampusphenols.z13.web.core.windows.net
Calculating Heat And Specific Heat Worksheets Answer Key Specific Heat And Calorimetry Worksheet Answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. calorimetry and specific heat calculation worksheet. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. chemistry 122 specific heat and calorimetry worksheet 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of. Specific Heat And Calorimetry Worksheet Answers.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat Calculations Worksheets Answers Specific Heat And Calorimetry Worksheet Answers The temperature of 335 g of water changed from 24.5oc to 26.4oc. specific heat and heat capacity worksheet. calorimetry and specific heat calculation worksheet. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. in this set of practice questions, we will go over the main types of questions on calorimetry. Specific Heat And Calorimetry Worksheet Answers.
From ar.inspiredpencil.com
Specific Heat Lab Calorimeter Specific Heat And Calorimetry Worksheet Answers in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry and specific heat calculation worksheet. specific heat and heat capacity worksheet. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. calculate the amount of heat. Specific Heat And Calorimetry Worksheet Answers.
From worksheetsirenij.z13.web.core.windows.net
Heat And Calorimetry Worksheets Specific Heat And Calorimetry Worksheet Answers chemistry 122 specific heat and calorimetry worksheet 1. calorimetry and specific heat calculation worksheet. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Q=mcδt (specific heat of. Specific Heat And Calorimetry Worksheet Answers.
From gekist4z3materialdb.z13.web.core.windows.net
Heat And Calorimetry Worksheets Specific Heat And Calorimetry Worksheet Answers calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. Find the equilibrium temperature if 200.0 grams of cold water. specific heat and heat capacity worksheet. The temperature of 335 g of water changed from. Specific Heat And Calorimetry Worksheet Answers.
From printablehalussw.z19.web.core.windows.net
Heat And Calorimetry Worksheets Specific Heat And Calorimetry Worksheet Answers The temperature of 335 g of water changed from 24.5oc to 26.4oc. chemistry 122 specific heat and calorimetry worksheet 1. specific heat and heat capacity worksheet. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Find the equilibrium temperature if 200.0 grams of cold water. = mc∆t, where q. Specific Heat And Calorimetry Worksheet Answers.
From imsyaf.com
Calorimetry Worksheet Answer Key Specific Heat And Calorimetry Worksheet Answers chemistry 122 specific heat and calorimetry worksheet 1. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. 1) if. Specific Heat And Calorimetry Worksheet Answers.
From afashionlista.blogspot.com
Answer Key Calorimetry Lab Gizmo Answers Activity C / Explore Learning Specific Heat And Calorimetry Worksheet Answers specific heat and heat capacity worksheet. ch 16 specific heat problems: A calorimeter has a heat capacity of 4.18 kj/g oc. Find the equilibrium temperature if 200.0 grams of cold water. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. 1) if 0.315 moles of. Specific Heat And Calorimetry Worksheet Answers.
From worksheetsirenij.z13.web.core.windows.net
Heat And Calorimetry Worksheets Specific Heat And Calorimetry Worksheet Answers calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of. specific heat and heat capacity worksheet. The temperature of 335 g of water changed from 24.5oc to 26.4oc. A calorimeter has a heat capacity of 4.18 kj/g oc. Find the equilibrium temperature if 200.0 grams of. Specific Heat And Calorimetry Worksheet Answers.
From zipworksheet.com
Calorimetry Worksheet Answer Key Specific Heat And Calorimetry Worksheet Answers specific heat and heat capacity worksheet. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. A calorimeter has a heat capacity of 4.18 kj/g oc. chemistry 122 specific heat and calorimetry worksheet 1. Find the equilibrium temperature if 200.0 grams of cold water. calculate the amount of heat transferred from the engine to the surroundings. Specific Heat And Calorimetry Worksheet Answers.
From escolagersonalvesgui.blogspot.com
Chemistry Worksheet Heat And Calorimetry Problems Escolagersonalvesgui Specific Heat And Calorimetry Worksheet Answers Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. A calorimeter has a heat capacity of 4.18 kj/g oc. specific heat and heat capacity worksheet. calorimetry and specific heat calculation worksheet. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. The temperature of 335 g of water changed from. Specific Heat And Calorimetry Worksheet Answers.
From ame.my.id
Calculating Specific Heat Worksheet Ame.my.id Specific Heat And Calorimetry Worksheet Answers A calorimeter has a heat capacity of 4.18 kj/g oc. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. chemistry 122 specific heat and calorimetry worksheet 1. ch 16 specific heat problems: calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of. 1). Specific Heat And Calorimetry Worksheet Answers.
From evon17vvdmaterialdb.z13.web.core.windows.net
Specific Heat And Calorimetry Worksheets Specific Heat And Calorimetry Worksheet Answers calorimetry and specific heat calculation worksheet. chemistry 122 specific heat and calorimetry worksheet 1. Find the equilibrium temperature if 200.0 grams of cold water. specific heat and heat capacity worksheet. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. ch 16 specific heat. Specific Heat And Calorimetry Worksheet Answers.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets Specific Heat And Calorimetry Worksheet Answers chemistry 122 specific heat and calorimetry worksheet 1. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. calculate the amount of heat. Specific Heat And Calorimetry Worksheet Answers.
From www.abhayjere.com
Calorimetry Worksheet Answer Key Specific Heat And Calorimetry Worksheet Answers in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry and specific heat calculation worksheet. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Find the equilibrium temperature if 200.0 grams of cold water. 1) if 0.315 moles of. Specific Heat And Calorimetry Worksheet Answers.